Introduction
Isogeometric Analysis (IGA) has recently emerged as a cost-effective alternative to classic isoparametric Finite Element Analysis. Computational biomechanics is a natural application of IGA for the following reasons:
- IGA implies the ability to describe exactly the computational domain geometry throughout the analysis process. This property can be exploited for the creation of patient-specific anatomical geometries and devices;
- The smoothness and the optimal continuity control of IGA basis functions lead to many advantages in the management of problem non-linearities, i.e. contact mechanics and nearly incompressible materials.
Aim
A novel computational framework is proposed in order to get IGA-suitable geometries to simulate different vascular minimally-invasive procedures. The preliminary results show the capability of the framework to import in a straightforward way patient-specific vascular geometries and set an analysis environment suitable for the solver FEAP.
Materials and Methods
The present work aims to provide, given a set of vascular medical images, a geometrical structure IGA-suitable. This task can be achieved through the following steps:
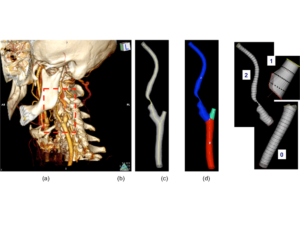
- Image processing: the set of DICOM medical images need to be processed in order to get a .nite number of point able to describe the vascular surface;
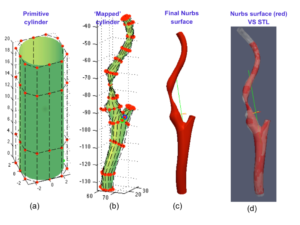
- Mapping: starting from a given NURBS parametrization (primitive surface) and the set of points previously extracted (reference surface), a least-square based mapping procedure was implemented in order to obtain two patient-specific NURBS surfaces, resembling the inner and outer vascular wall, respectively;
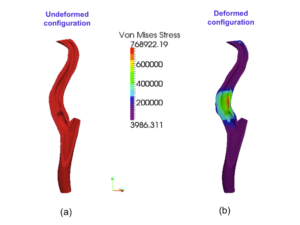
- FEAP preliminary analysis: as preliminary analysis, a simulated angioplasty (AP) procedure for the carotid artery has been performed. From the clinical perspective AP is de.ned as the technique of mechanically widening narrowed or obstructed arteries, typically being a result of atherosclerosis. This task is achieved by mean of a ballon catheter that is passed into the narrowed location and then in flated to a fixed size and subsequently deflated and withdrawn.
Conclusions
In the present study we present a novel computational framework able to integrate medical information and computational tools in order to perform IGA for vascular biomechanics. The results suggest that it is possible to obtain an IGA-suitable structure in a reasonable number of steps. Moreover, a preliminary analysis has been performed in order to make the point about the capability of the workflow to reproduce, in a simplified way, a minimally-invasive procedure present in the clinical practice. In order to obtain a valuable tool for a wider range of clinical procedures, such as stenting and grafting, some more sophisticated components (such as stable contact driver, pressure elements, biologic materials constitutive models etc.) need to be developed. We think that IGA for vascular biomechanics represents a big challenge both from computational and model design viewpoint and can give, in the next future, a crucial contribution to the integration of medicine and numerical analysis.
Main collaborations
- Department of Civil Engineering,University of California at Berkeley, Prof. R.L. Taylor
- Technische Universitat Braunschweig, Germany, Prof. L. De Lorenzis