Background
Modeling soft biological tissues as anisotropic materials requires the definition of a proper collagen fibers architecture. Anisotropy prediction in tube-like arterial models is very simple and based on experimental observations that two families of collagen fibers are embedded in an isotropic ground matrix and have a mean helical orientation around the circumferential direction [R1, R2]. Anisotropy in complex geometries may be introduced either calculating the principal stress directions from an isotropic analysis, setting fiber directions at an angle between the first and second principal directions of stress [R3, R4]; or defining a proper local coordinate system in each element of the (reference) FE mesh, setting fiber directions at an angle between the circumferential and axial directions [R5, P1].
Goals and Methods
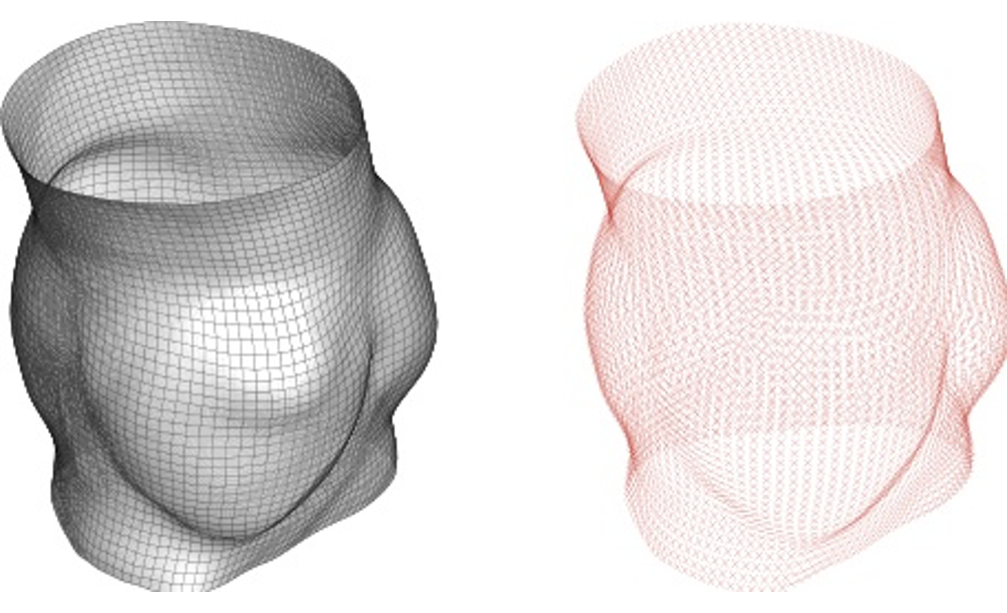
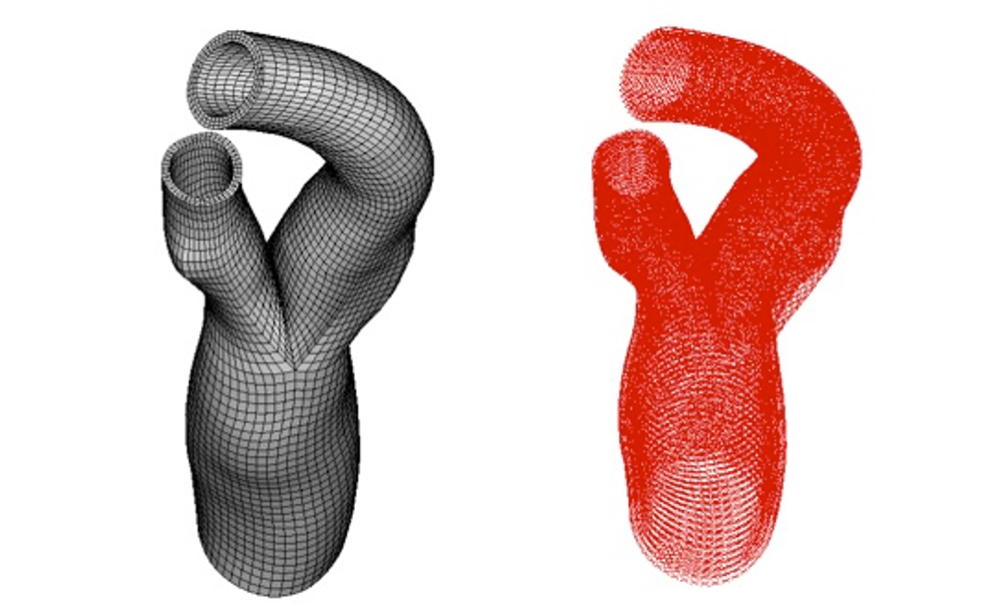
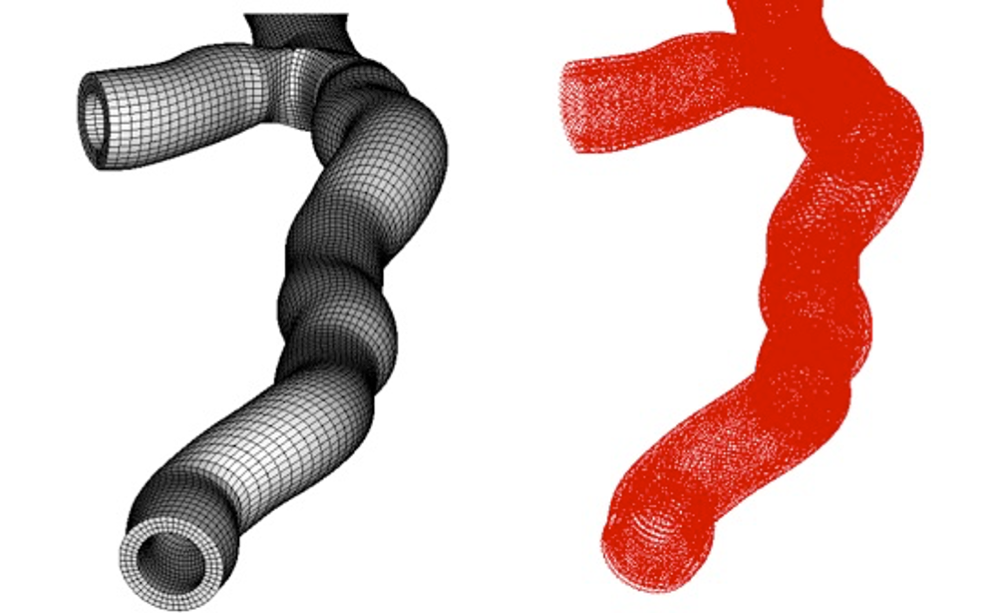
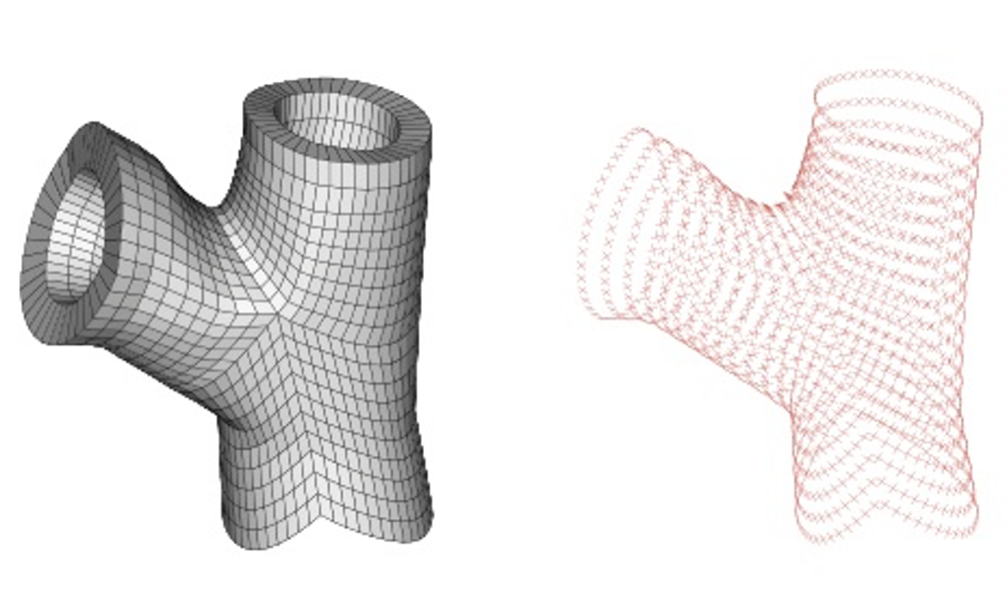
The goal of this study is to define the collagen fibers architecture in complex geometries, such as patient-specific geometries of aortic valve root and of bifurcations in carotid and coronary arteries. The approach is a generalization to complex geometries of the method used in tube-like arterial model, wherein a local cylindrical coordinate system is defined for each element of the FE mesh with the local axial axes coincides with a segment of the centerline.
Results
References
[R1] J. D. Humphrey, Cardiovascular solid mechanics. Cells, tissue and organs, SpringerVerlag, New York, 2002.
[R2] N. J. B. Driessen, W. Wilson, C.V.C. Bouten and F.P.T. Baaijens. A computational model for collagen fibre remodelling in the arterialwall. Journal of Theoretical Biology 226 (2004) 53–64.
[R3] Hariton, G. deBotton, T. C. Gasser and G. A. Holzapfel. Stress driven collagen fiber remodeling in arterial walls. Biomechanics and Modeling in Mechanobiology 6 (2007) 163–175.
[R4] V. Alastrue, A. Garcia, E. Pena, J. F. Rodriguez, M. A. Martinez and M. Doblare. Numerical framework for patient-specific computational modelling of vascular tissue. International Journal for Numerical Methods in Biomedical Engineering 26 (2010) 35–51.
[R5] P. Mortier, G.A. Holzapfel, M. De Beule, D. Van Loo, Y. Taeymans, P. Segers, P. Verdonck and B. Verhegghe. A novel simulation strategy for stent insertion and deployment in curved coronary bifurcations: comparison of three drug-eluting stents. Annals of Biomedical Engineering 38 (2010) 88–99.
Group publications
[P1] F. Auricchio, M. Conti, A. Ferrara, S. Morganti and A. Reali. Patient-specific finite element analysis of carotid artery stenting: a focus on vessel modeling. International Journal for Numerical Methods in Biomedical Engineering. 29 (2013) 645–664.