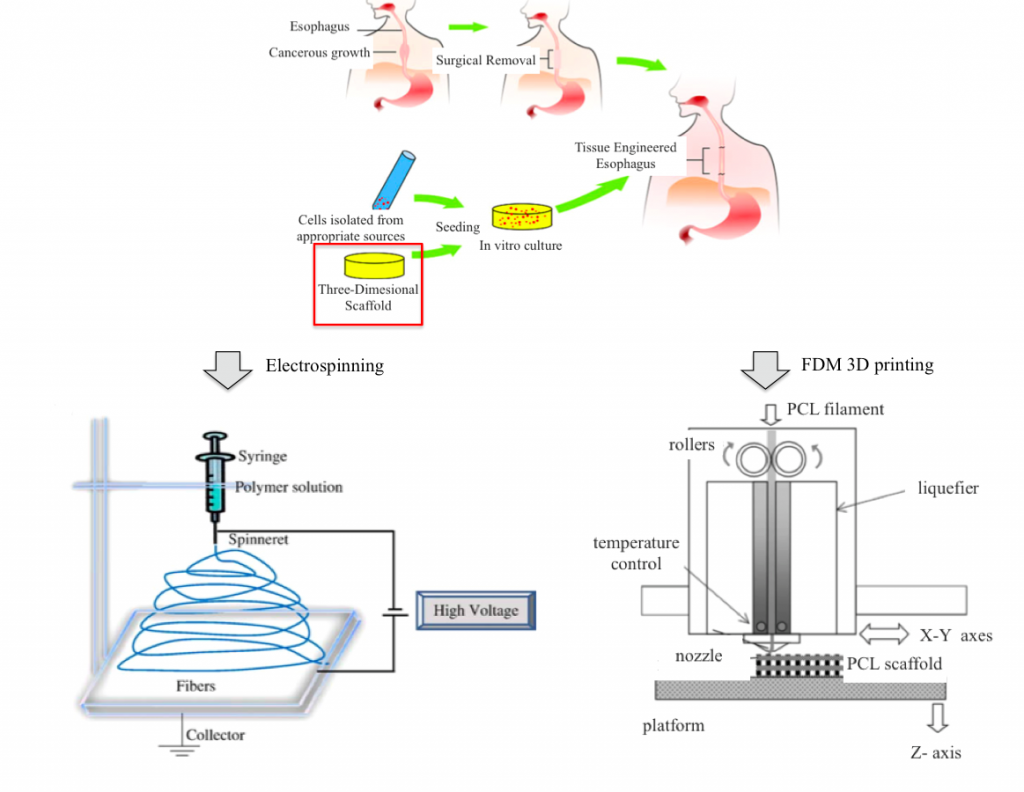
Introduction
Every year about 5,000–10,000 patients are diagnosed with esophageal diseases for which esophageal replacement is needed [Maghsoudlou et al., Tissue engineering of the esophagus, Seminars in Pediatric Surgery, (2014)]. Current choices for esophageal replacement involve the use of more distal parts of the gastrointestinal tract or colon, with long-term problems associated to these technique – such as graft failure, recurrent strictures, retarded voice rehabilitation and lifelong immunosuppression therapy. In view of such limitations, the immense potential offered by tissue engineering has been recognized and can be exploited as a good alternative for esophageal replacement. An esophageal scaffold must provide the necessary features – such as porosity, pore size and surface chemistry – to facilitate cell attachment and proliferation and the mechanical properties suitable to accomplish esophageal tissues function. Two biocompatible polymers have shown promising results for esophageal tissue engineering and for the creation of biocompatible and biodegradable patches for esophageal injuries: polylactic acid (PLA) and polycaprolactone (PCL). Scaffolds and patches can be prepared by solvent casting or electrospinning techniques.
Idea
The project comes from the collaboration among the 3D printing laboratory ProtoLab of the Civil Engineering and Architecture Department (DICAr) and the Department of Pharmaceutical Sciences of the University of Pavia. The aim is to explore the potential of 3D printing technology on scaffold manufacturing in order to produce customized esophageal patches.
The idea is to print via FDM process co-polymeric PLA-PCL esophageal patches and to compare them with patches synthesized via solvent casting method in the Pharmaceutical Sciences lab. Solvent casting method is a powder blend technique for patches production through solubilization, freezing and lyophilization cycles.
3D printing allows to reproduce biological/geometric characteristics – such as dimensions and pores size – and mechanical properties of patches synthesized by our partners, drastically reducing production time and paving the way to innovative solutions tailored on the patient specific clinical case.
Both types of patches are subjected to biological characterization (cell adhesion and proliferation tests), mechanical characterization (tensile tests), physical-chemical and morphologic characterization (GPC, DSC, SEM) to compare the preparation techniques and their results. Conducted tests are:
- Gel Permeation Chromatography (GPC): determination of molecular weights of the used biopolymers before and after being processed.
- Differential Scanning Calorimetry (DSC): thermal analysis of polymers and copolymer before and after being processed.
- 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT assay): cell attacchement and proliferation analysis.
- Scanning Electron Microscope (SEM): evaluation of pore size, shape and distribution throughout the patches.
- Tensile tests: mechanical characterization of patches.