Introduction
Aorta is the main artery of human circulatory system. Aortic functionality can be impaired by number of cardiovascular diseases resulting often in aneurysm, i.e., an abnormal bloodfilled vessel dilatation or dissection, i.e., a pathological splitting of the aortic media layer. Despite traditional open-surgery remains the gold standard for treating aortic pathologies [R1], the use of minimally invasive endovascular techniques is rapidly arising in the recent years as a valuable alternative [R2]. In particular, thoracic endovascular aortic repair (TEVAR) is getting predominant for treating patient with acute dissections, descending thoracic aneurysms, and thoracic transactions [6]. TEVAR is a minimally-invasive procedure aiming at correcting the impaired haemodynamics diverging the blood flow through the endovascular apposition of a metallic frame covered by a Dacron skirt, called endo-graft (see Fig. 1). The simplicity of the prosthesis design is counterparted by complexity of procedure planning. In fact, many TEVAR drawbacks (graft dislocation, endoleaks, etc.) are still unsolved and most of them, ranging from poor radial force to post-implant migration, are due to biomechanical issues. Furthermore, there is still a poor treatment standardization because: 1) many devices with different mechanical properties and specific features are available in a fast growing market; 2) the geometry of the target vessel is highly patient-dependent, especially in a critical pathological cases.
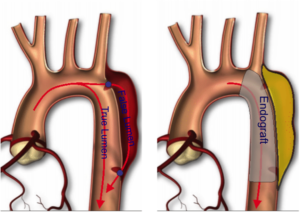
Fig. 1: Illustrative sketch of aortic dissection. The disease is characterized by a delimitation of the vessel wall, i.e., intimal tear, which triggers the intra-layer blood flow infiltration, promoting thus the creation of an alternative haemodinamical path, called false lumen, running parallel to native true lumen (see picture on the left). The insertion of an endovascular prosthesis, called endograft, restores the blood flow path (see figure on the right).
Goal
All the previous considerations call for a quantitative and standardised approach aimed at integrating different knowledge fields, such as medicine, mechanics, and fluid-dynamics. Given these premises, our research activity aims at developing and use computational tools, such as structural Finite Element Analysis (FEA), as valuable clinical support by predicting the performance of a given endovascular therapeutic option with respect a given patient-specific case.
Method
The analysis is based on: i) accurate image analysis aimed at reconstructing the pre-operative vascular lesion to treat; ii) accurate modeling of the prosthesis model based on the real prosthesis samples. Moreover, as proposed in P1, we have also compared the simulation prediction based on pre-operative images with postoperative outcomes for validation purposes (see Fig. 2).
Key clinical collaboration: IRCCS Policlinico San Donato (Milan)
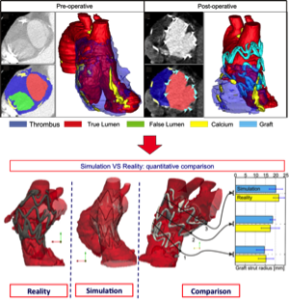
Fig. 2: Detail of fiber orientation in the bifurcation zone for each arterial layer.
References
- [R1] L. Hiratza, et al., 2010 ACCF/AHA/AATS/ACR/ASA/ SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease, Circulation 121 (2010) e226– 369.
- [R2] S. Scali, P. Goodney, W. et al.. D.B., National trends and regional variation of open and endovascular repair of thoracic and thoracoabdominal aneurysms in contemporary practice, J Vasc Surg 53 (2011) 1499–1505.
Group publications
- [P1] F. Auricchio, M. Conti , S. Marconi, A. Reali, J. Tolenaar, S. Trimarchi. Patient-specific aortic endografting simulation: from diagnosis to prediction . Computers in Biology and Medicine, 43(4): 386-394 (2013).

